Dear Patients of Sansum Clinic,
Once again, the COVID-19 pandemic is changing. Now, there is a new variant on the scene – the so called “Omicron” variant – which is driving up cases internationally, nationally and locally. I should comment that we don’t have a lot of local sequencing data, which is how we know what variant is circulating, but we know it is here in the US and we believe it is here locally – in Santa Barbara. This variant may not be any more lethal for an individual person than those that came before, though the verdict is out on that still. But, it is indisputably more infectious than those variants that proceeded it. Data supports that and, likely, your own experience of knowing someone who has recently been infected or knowing of a cluster of cases may have told you that something different is going on. Even if Omicron is slightly less virulent than Delta for an individual person, if it infects vastly more people, the impact will feel as if it is more virulent in terms of its effect on a community, particularly the unvaccinated part of the community.
Dr. Marjorie Newman, the Medical Director at Sansum Clinic, sends out weekly updates to our staff (including our doctors). It covers everything you likely want to know about the current state of affairs and so, again, I’m including that update below.
Some themes touched on before are still true and worth repeating.
- Vaccines work. They are not perfect and you may still get infected even if you are fully vaccinated and even if you have received a booster. But the chances of getting severely ill and hospitalized and the chance of dying as a result of COVID-19 are markedly reduced in those who are vaccinated and particularly in those who have received a booster dose.
- Therapeutics continue to evolve. The new Omicron variant may not be as susceptible to some of the existing monoclonal antibody treatments as its predecessor variants. That is a not-so-good development. On the positive side, the FDA issued an EUA (Emergency Use Authorization) for its new oral (pill) treatment of COVID-19. The medication is not yet available in pharmacies, but with the approval it will begin to be distributed soon. It has specific requirements for its use and has many drug-drug interactions, but it is the start of a very positive development.
- Sansum Clinic staff have, like other healthcare providers, worked tirelessly for almost two years. The other evening, we were doing a night-time vaccine clinic and there were nurses enthusiastically vaccinating community members after working all day in their day jobs. The patients were delighted to be getting vaccinated before the impending Omicron invasion. Even in a pandemic you see some good things happen.
Below is Dr. Newman’s excellent summary of all things COVID.
Even though the pandemic is not yet gone, have a happy and safe Holiday.
Kurt Ransohoff, MD, FACP
CEO, CMO

Colleagues
From: Marjorie Newman, MD
Re: Weekly COVID-19 Update-Cases are surging and Omicron is now the predominant variant in the U.S.
Overview: The U.S. Coronavirus case levels continue to rise, now with over 154,000 new cases reported today (up from ~121,000 new cases per day on average from the prior week) and up 27% from the prior 14 day average with hospitalizations at 69,200 (up 20% in the past 14 days), and the deaths are up to 1,300 per day which is a 4% rise in the past 14 days. Conditions are worsening significantly in the Northeast where hospitals are again getting overloaded with critically ill COVID patients. Yesterday the CDC reported that the Omicron variant now accounts for nearly 75% of all new COVID-19 infections, overtaking the Delta variant, which highlights how incredibly infectious the Omicron variant is. In just three weeks the Omicron variant went from accounting for 3% to now nearly 75% of all new COVID-19 infections in the U.S.
The two dose mRNA vaccines, along with getting a booster dose when eligible, remains very effective at preventing severe cases and death as a result of COVID-19, whether due to the Delta or the Omicron variant, and that is why it is still so important to encourage everyone to get vaccinated and to get a booster when eligible. Booster doses have been shown to provide considerable protection against waning immunity that has been noted several months after completing the two dose series of an mRNA vaccine and also provides added protection against infection with the highly transmissible Omicron variant.
Below is a graph from the Johns Hopkin’s COVID -19 Resource Center which depicts the case rate (7 day moving average) for the 10 most impacted countries. The U.S. (light green trend line) case rate, continues to show a steep rise in cases and there is great concern regarding the highly transmissible Omicron variant combined with the holiday travel season which is already underway. Several European countries are continuing to also see a steep rise in cases fueled by the Omicron variant, with the UK now at 80,000 cases per day, Germany at 40,000 and France at 48,000 cases per day. Globally, since the pandemic started, there have been 273 million cases of COVID-19 and over 5.34 million deaths. In the U.S. since the pandemic started there have been 50.6 million cases and sadly we have surpassed 806,000 total deaths due to COVID-19, which is the greatest number of reported deaths of any country, with individuals age 65 and older making up three quarters of those who have perished. The unvaccinated in the 65-79 years age group are at a risk 30 times higher than those who are vaccinated, again underscoring the importance of vaccinations (see graph below re: COVID-19 weekly death rate by vaccination status from the CDC/MMWR).
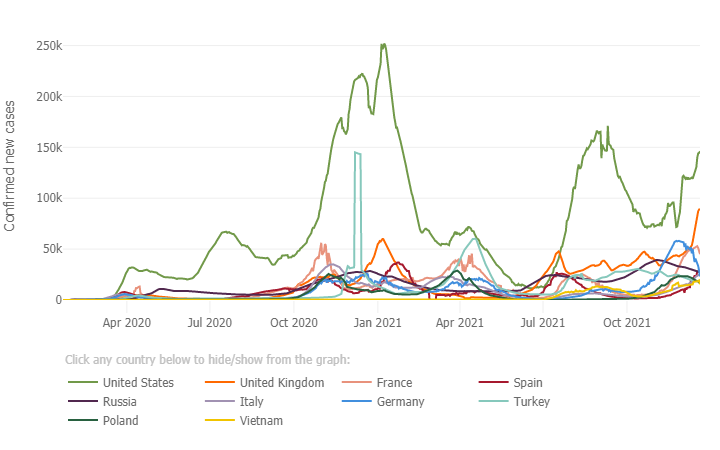
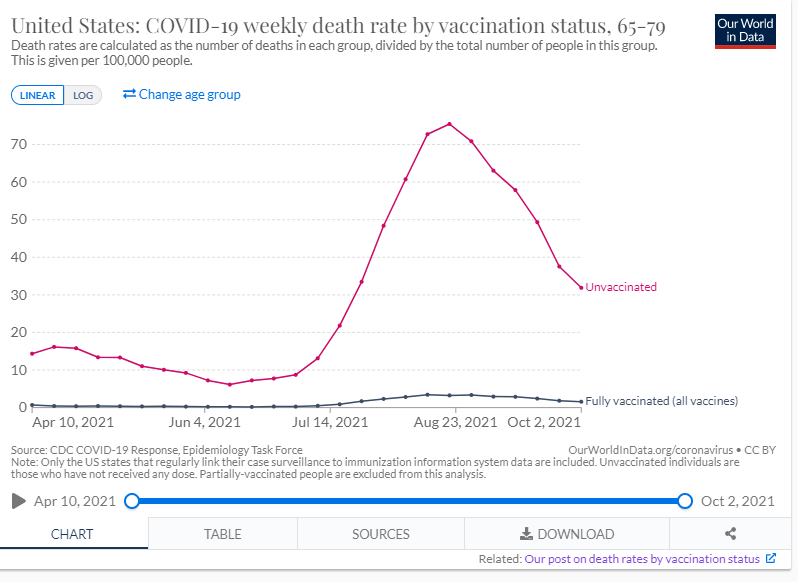
In the US, ~1.62 million doses of vaccine are being administered each day, including first, second and additional doses. As of December 18th 65% of individuals age 5 and older are fully vaccinated; 72% of all adults in the U.S. age 18 and older are fully vaccinated, 87% of adults age 65 and up are fully vaccinated and 61% of all individuals in the U.S. are fully vaccinated.
Omicron FAQs
How contagious/transmissible is the Omicron Variant? Omicron is significantly more contagious than Delta, with an estimated doubling time of 2 days, whereas Delta had a doubling time of 4.5 days, and the incubation time for Omicron is estimated to be 3 days, shorter than the incubation time for the prior variants. Additionally Omicron is better at evading immunity generated by vaccines or by natural immunity from previous infections, although vaccines/boosters sill provide protection against severe illness and death. As mentioned above, as of two weeks ago Omicron accounted for only 3% of the COVID-19 cases in the US, last week it accounted for 13% of all cases in the U.S and as of yesterday, it accounts for nearly 75% of all COVID cases in the U.S. and has supplanted the Delta variant as the predominant variant in the U.S.
Where has Omicron been detected in California? As we know, Genomic sequencing data can lag behind PCR testing data, given the time it takes to perform genomic sequencing. However, Omicron has been detected in many counties throughout California, and given the surging case rates it is anticipated that it will soon be the dominant variant in CA, if not already, as it is in the rest of the US.
Is the Omicron variant in SB County? Although it hasn’t been sequenced yet because sequencing of a virus can take several weeks, this variant is most likely already in Santa Barbara County especially with the rising case rates that we are seeing over the past few days and sequenced/confirmed Omicron cases in counties to the north and south of us.
Can I get the Omicron Variant if I am vaccinated and boosted?
Yes, given the high transmission rate of the Omicron variant, it is possible that individuals who have been vaccinated and boosted can get infected, but the good news is that the infection is likely to be less serious and not result in severe illness, hospitalization or death. According to the CDC and CDPH, there have been cases where individuals who were fully vaccinated and received a booster were infected by the omicron variant. However, current vaccines are expected to protect against severe illness, hospitalizations, and deaths due to infection with the Omicron variant. However, breakthrough infections in people who are fully vaccinated are likely to occur. The CDC is advising people to get fully vaccinated and boosted to slow transmission and reduce the chance of new variants emerging. Booster shots with either Pfizer or Moderna, will result in immediate increases in antibodies which will continue to protect you from severe disease.
Can I get the Omicron Variant if I had a prior natural infection? Yes, data from South Africa demonstrated that natural immunity as a result of a prior COVID infection waned over a matter of months and individuals were getting re-infected with the Omicron variant.
If I can still get infected despite getting vaccinated and boosted, should I still get vaccinated? Absolutely YES, because vaccination followed by a booster dose 6 months later has been shown to increase protective antibody levels against Delta and Omicron variants and will protect you from getting severe disease, which can result in hospitalization or death.
Is there any greater clarity on the severity of illness with Omicron? While there are early reports from South Africa that suggests Omicron might cause less severe illness than the Delta variant, there has not been enough data or case load to make any definitive determinations about the severity of illness as there are many factors that can confound the early findings. Although South Africa has reported far fewer hospitalizations from omicron compared to previous waves, the UK is in the midst of a sharp rise in hospitalizations, about 30 percent higher week over week, which is raising concerns around the globe. We know with COVID-19 infection the illness can progress over a matter of weeks leading to hospitalization and death several weeks after the initial infection. As a result more time is needed to see how virulent Omicron will be and how it will impact local health care communities. Factors that will influence severity of illness, such as vaccinations and boosters, remain the most important tools we have to prevent hospitalizations and deaths from COVID-19. Even if Omicron is less severe than the Delta variant, if enough people are infected, hospitals and health care systems will likely be overwhelmed just due to the greater number of individuals who are likely to get infected. As a result, hospitals and health care systems nationwide, many of which are already short staffed, have expressed concerns about the added strain that this surge will place on healthcare resources. Consequently, it is incredibly important to invoke all mitigation measures, like getting vaccinated, getting a booster when eligible, masking in indoor public settings, avoiding large gatherings, etc.,
What should I do if I test positive for COVID-19? Isolation and quarantine guidance remains the same for Omicron, at least for now. If you test positive, you should immediately isolate away from others and isolate for a duration of 10 days from symptom onset (or 10 days from a positive test, if asymptomatic), to avoid infecting more people. If your symptoms persist past 10 days (e.g., continued fever, cough, etc.), you should remain in isolation and contact your provider for further guidance. See attached isolation and quarantine instructions which have not changed since May 2021.
What should I do if I am a close contact of someone who tests positive?
If you have been in close contact with an infected person, if you are fully vaccinated, you do not need to quarantine unless you develop symptoms, However, even if asymptomatic you should get tested within 3-5 days after exposure and then again in 5-7 days, to ensure that you remain negative. Always wear a mask indoors in public for 14 days following exposure. Attached to the e-mail is the most current isolation and quarantine guidance from the CDPH and additional information can be found at the below link.
California Update:
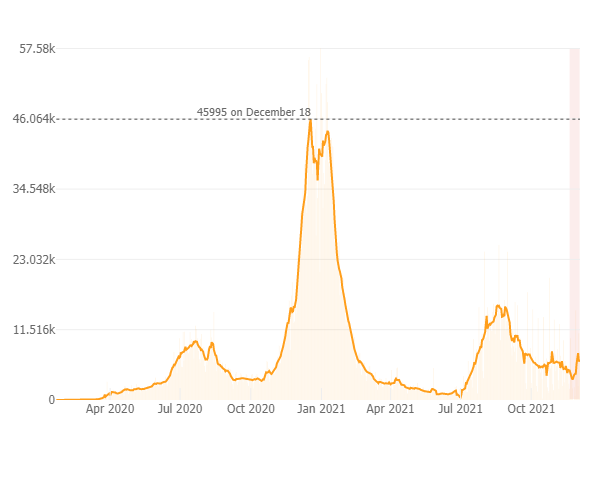
As of December 21st since the pandemic began there has been 4.9 million positive COVID-19 cases reported in California; Over 75,000 deaths and 62.7 million vaccines administered. The daily case rate throughout California continues to rise and is now at 14 new cases per 100K (daily average has increased to 5,300 cases per day in past week), and a test positivity rate of 2.8 % with 3,900 people hospitalized throughout the state and 117 new deaths in the past week. The graph below from the Johns Hopkins COVID-19 Resource website depicts the rising case count in California with the light pink shaded area indicating that the case rate has increased over the prior two weeks after having declined for several weeks prior. In CA 70 % of all individuals are fully vaccinated and 8% are partially vaccinated.
As a result of the rising case rates an indoor masking mandate for indoor public spaces in all counties in California was implemented last week and will be in effect until mid-January at which time it will be reassessed.
Santa Barbara County:
According to the Santa Barbra County Public Health Department website, as of Tuesday December 21st there are currently 512 active infections with 67 new cases reported yesterday. The test positivity rate in SB county is 3.7% with a rising case rate, reported by the CDC to be 106 cases per 100K. There are now 31 patients in the hospital and 560 deaths in Santa Barbara County due to COVID-19 since the onset of the pandemic.
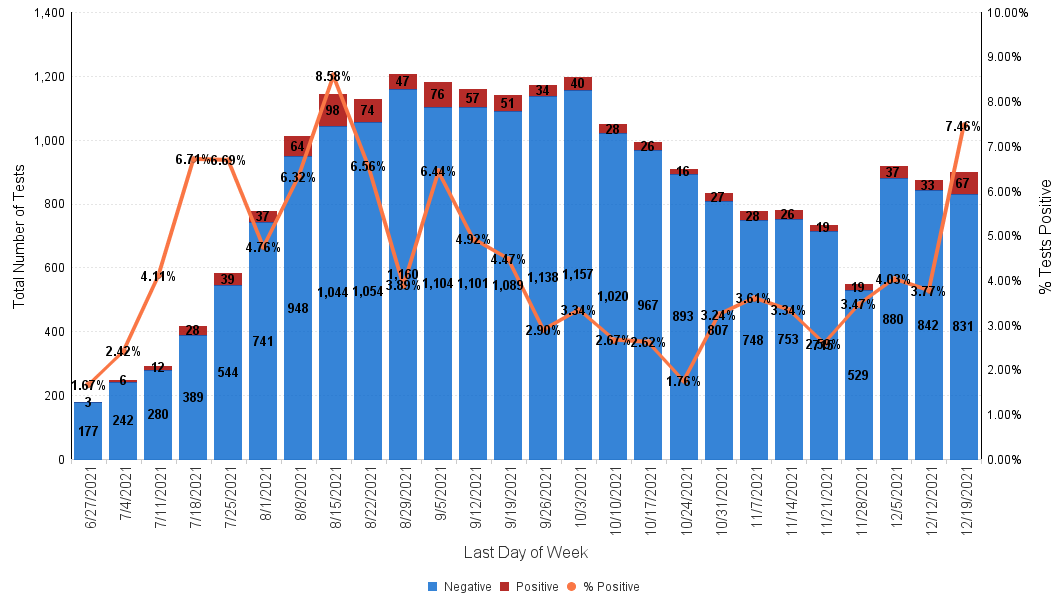
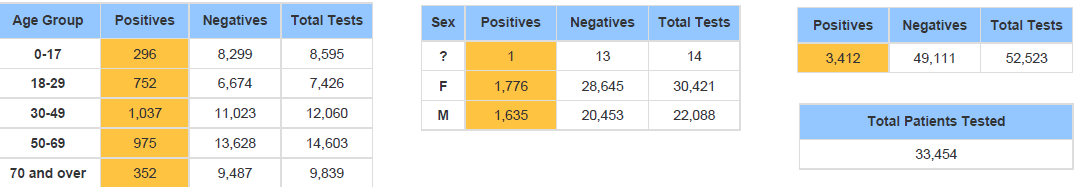
Below is the Sansum Clinic weekly COVID-19 testing data for the week ending December 19th. In line with what is being seen in the rest of California and throughout the county, our percent positive has steeply increased over the past few days and has doubled from 3.72% last week to 7.46%, with the greatest increase in cases evenly distributed in the 18-69 age ranges.
COVID-19 Vaccinations in Santa Barbara County: As of December 21st 688,676 vaccines have been administered, with 68.4 % of those eligible to receive vaccine (age 5 and up) are now fully vaccinated and 64.3 % of the county (all ages) fully vaccinated.
________________________________________
UPDATES & REMINDERS:
COVID Vaccines:
- As of last week the CDC Advisory Panel is recommending mRNA vaccines over the Johnson &Johnson vaccine due to vaccine efficacy, safety and rare side effects related to the J&J vaccines (blood clotting in women age 18-49 noted in 8.7 cases per 1 million shots administered and deaths in 1.2 of those cases). The CDC advisory panel voted unanimously to recommend that people preferentially use COVID-19 mRNA vaccines, shots made by Moderna and Pfizer-BioNTech, other than Johnson & Johnson's single-shot dose vaccine. Individuals who are unable or unwilling to receive an mRNA vaccine will continue to have access to Johnson & Johnson’s COVID-19 vaccine.
- Pfizer’s pediatric vaccine for children 6 months to under 5 years, which was one tenth of the adult dose (3 micrograms) and given as a two dose series, did not provide the expected immunity in children in this age range and as a result, Pfizer will be adding a third dose to the regimen to be given at least two months after the second dose. No safety concerns were noted and ongoing studies will be performed to determine immune response to the third dose. We will keep you posted as this evolves but at this time there is no vaccine approved for children under age 5 years..
Booster Update/Reminder:
- Last week Pfizer/BioNTech announced that a booster dose of their vaccine increased protection significantly against waning immunity against the Delta variant and also significantly increased neutralizing antibody levels against the Omicron variant.
- In an announcement early yesterday, Moderna said preliminary data from lab testing found the version of its booster currently in use in the United States and elsewhere provided increased antibody levels to neutralize the Omicron variant and it also found that a double dose of the booster shot provided a much greater increase in those levels. The drug company said its currently FDA-approved 50 microgram booster was found to increase neutralizing antibody levels against omicron 37-fold compared to pre-boost levels. Meanwhile, it found that a 100 microgram booster dose gave an 83-fold increase in neutralizing antibody levels.

Long-acting Monoclonal Antibody Treatment for Prevention of COVID-19 for those who are Immunocompromised: As discussed last week, the FDA issued an emergency use authorization of a monoclonal antibody treatment developed by AstaZeneca, Tixagevimab/cilgavimab, with a brand name Evusheld, which is along acting antibody treatment for individuals (12 years and older who weigh at least 40kg), with weakened immune systems to be used as pre-exposure prophylaxis in high risk individuals not expected to respond to vaccines and can also be used in patients for whom vaccines are not recommended because they are allergic to the vaccine ingredients. The medication is administered as two separate sequential intramuscular injections. Studies demonstrated that Evusheld reduced the risk of developing symptomatic COVID-19 by 77% compared to placebo and the treatment was effective for at least 6 months. However, one caveat is that this study was performed prior to the emergence of the Omicron variant and it is not clear how effective Evusheld will be against this variant. In addition this medication is in very limited supply and is being distributed in very small quantities. Sansum Clinic anticipates receiving a small allotment of this therapy from the California Department of Public Health (CDPH) to offer judiciously to those who are severely immunocompromised, such as transplant patients.
Monoclonal Antibody Therapy Update: This morning the CDPH indicated that the effectiveness of the current monoclonal antibody therapies, Bamlanivimab/etesevimab and Casirivimab/imdevimab, for those who are ill with COVID-19 and are at high risk for severe illness, may not be effective against the Omicron variant as a result of the many mutations on the spike protein of the Omicron variant. In vitro studies demonstrated that the monoclonal antibody therapies lost neutralizing activity when tested against the Omicron variant. However, the monoclonal antibody Sotrovimab, which targets areas of the spike protein which have remained constant, appears to retain activity against the Omicron variant. Sotrovimab is an IV infusion and is indicated in individuals who are COVID-19 positive and are at risk of severe illness as a result of underlying medical conditions. It is not indicated in anyone under age 12 and is not approved for post exposure prophylaxis. Unfortunately there is limited supply of Sotrovimab but more doses are anticipated to be shipped regionally and ultimately more will be manufactured in light of the current Omicron surge.
COVID-19 Antiviral pill (Paxlovid) for high risk patients (age 12 and older): The FDA just issued an emergency use authorization for the antiviral oral medication, Paxlovid, made by Pfizer. Paxlovid has been found to be highly protective against severe illness in those who are at high risk of becoming severely ill from a COVID-19 infection due to age or comorbidities, such as obesity, diabetes, underlying pulmonary disease, etc. Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) can be prescribed for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms or about 88 pounds) with positive results of direct SARS-CoV-2 testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid will be made available by prescription only and should be initiated as soon as possible after diagnosis of COVID-19 and within five days of symptom onset.
Paxlovid consists of nirmatrelvir, which inhibits a SARS-CoV-2 protein to stop the virus from replicating, and ritonavir, which slows down nirmatrelvir’s breakdown to help it remain in the body for a longer period at higher concentrations. Paxlovid is administered as three tablets (two tablets of nirmatrelvir and one tablet of ritonavir) taken together orally twice daily for five days, for a total of 30 tablets. Paxlovid is not authorized for use for longer than five consecutive days. Possible side effects of Paxlovid include altered or impaired sense of taste, diarrhea, increased blood pressure, and muscle aches. In addition Paxlovid may interact with other medications due to the mechanism of action as a CYP3A inhibitor. Patients receiving medications metabolized by CYP3A may have increased plasma concentrations of these medications when taken with Paxlovid and dose adjustments may be indicated.
The primary data supporting this EUA for Paxlovid are from a randomized, double-blind, placebo-controlled clinical trial studying Paxlovid for the treatment of non-hospitalized symptomatic adults with a laboratory confirmed diagnosis of SARS-CoV-2 infection. Patients were adults 18 years of age and older with a prespecified risk factor for progression to severe disease or were 60 years and older regardless of prespecified chronic medical conditions. All patients had not received a COVID-19 vaccine and had not been previously infected with COVID-19. The main outcome measured in the trial was the proportion of people who were hospitalized due to COVID-19 or died due to any cause during 28 days of follow-up. Paxlovid significantly reduced the proportion of people with COVID-19 related hospitalization or death from any cause by 88% compared to placebo among patients treated within five days of symptom onset and who did not receive COVID-19 therapeutic monoclonal antibody treatment. It is expected that Paxlovid will also work well against the Omicron variant because the method of action is not dependent upon the spike protein. Paxlovid is not authorized for the pre-exposure or post-exposure prevention of COVID-19 or for initiation of treatment in those requiring hospitalization due to severe or critical COVID-19. Initial supplies of Paxlovid will be limited and may not be available for several weeks. As we know, Paxlovid is not a substitute for vaccination and should not be used in patients with severe kidney or liver impairment. Again, vaccination, along with a booster dose, is the best way to protect against severe illness for all who are eligible to receive a vaccine and booster dose.
Holiday Gatherings:
What preventive strategies should be employed to protect individuals from getting infected, especially during this holiday? The CDC recommends people follow prevention strategies such as wearing a mask in public indoor settings regardless of vaccine status (and optimize mask fit and filtration), especially in areas of substantial or high community transmission (like in CA, in which masking is now mandated in public in-door spaces), washing hands frequently, and physically distancing from others. CDC also recommends that everyone 5 years and older protect themselves from COVID-19 by getting fully vaccinated. In addition, if you are going to be gathering with friends and family for the holidays-consider performing a rapid COVID test 1-2 days prior to the event regardless of vaccination status to ensure that you don’t have a COVID infection that you may be able to spread to others, AND get tested 3-5 days after the event to ensure that you remain negative. CDC is also encouraging a COVID-19 vaccine booster dose for all those who are eligible.
We realize how draining these past two years have been for everyone, both in and out of healthcare, and we realize how disheartening it is to see cases surging again. We will get through this as we have with prior challenges. Working with all of you throughout the pandemic highlights how fortunate we are to have such a wonderful team of health care professionals at Sansum Clinic. We are so appreciative of the great care and compassion provided to our patients and the community and for the incredible team work that has allowed that to happen despite these trying times. Thank you for all you do! Wishing you all a wonderful, safe and happy holiday!
And as always, please don’t hesitate to reach out for any questions.
Marjorie Newman MD
Medical Director
Sansum Clinic